Modified lactides promise new implementations in pharmacology and catalysis
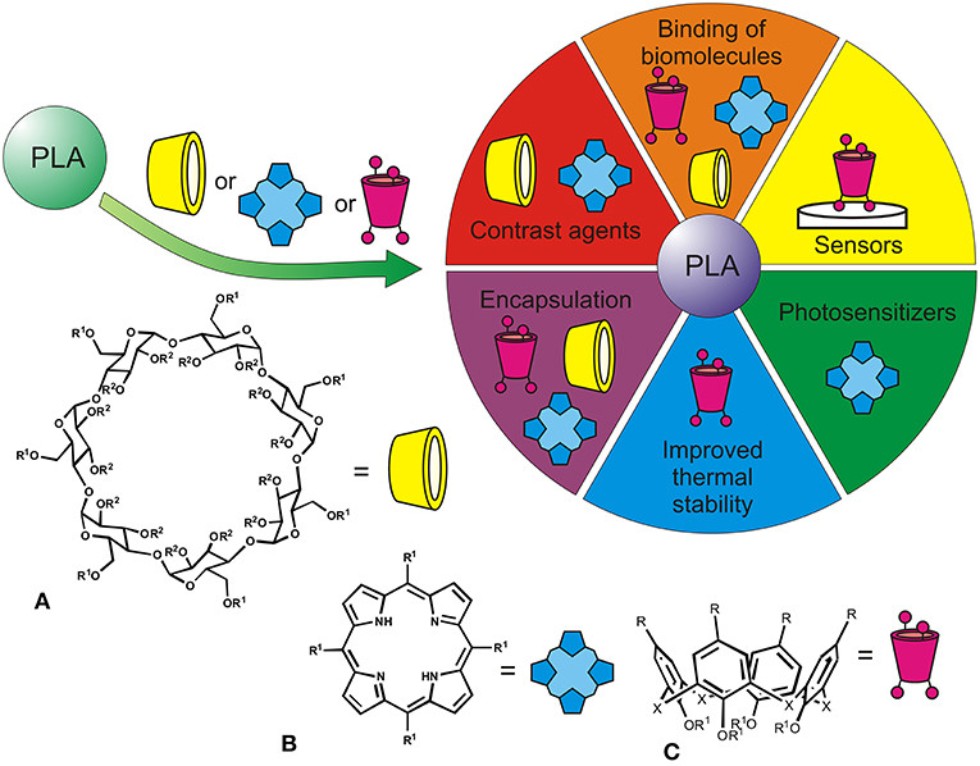
Figure 1. Applications of PLA functionalized with macrocyclic structures [β-cyclodextrins (A), tetrapyrroles (B), calixarenes (C), R1, R2 indicate possible modification by PLA fragments].
A paper was published in Frontiers in Chemistry.
The project concerning modification of oligo- and polylactic acids with thiacalix[4]arene derivatives was launched at Kazan Federal University three years ago. The main idea of the study was to improve physical and chemical properties of polymers by introducing macrocyclic rigid structures which determine spatial arrangement of linear chains of lactides in their microenvironment.
During this time, as the team leader, Professor Ivan Stoikov, comments, new synthetic approaches have been developed. The scientists have managed not only to obtain various oligomeric structures with variable length of the polymer fragments but also implement macrocycles with different arrangements of the functional groups in the product composition. Such macrocycles were called “macrocyclic knots” because of their likeness to fishing nets. As the research went on, it became clear that the introduction of the macrocycle solved problems that were above and beyond those stated in the initial plans.
In particular, regulation of the chain flexibility, internal space, and ability to self-assemble made it possible to obtain hybrids with inclusion of biopolymers, in which nucleic acids and proteins retained their native structure and biochemical functions, but also were separated in accordance with their effect on the polymer aggregation. As a result, such hybrids were successfully applied in prototypes of chemical and biochemical sensors, and it seems that they offer good prospects in creating new “smart” drugs and systems for targeted drug delivers. The team that works on this topic comprises two laboratories, i.e., it unites chemists who obtain new modified oligo- and polylactides and analysts who develop new enzyme and DNA sensors based on the compounds described above. Dr. Stoikov is in charge of the work.
Polycondensation products of lactic acid have been previously used in various products due to their low cost, chemical and biological inertness, non-toxicity and biodegradability. The project diversified these properties by imparting reception properties related to the modification of polylactides by macrocycles.
Modification products retain their advantages mentioned in the initial polymers, primarily biological inertness and chemical resistance, but are able to recognize some important specimens interesting for biomedicine and pharmacy, mostly proteins and nucleic acids. In turn, the hybrids formed in binding biopolymers become sensitive to biochemical interactions with drugs, toxins, etc. To achieve this, it was necessary to develop new methods for the synthesis of modified oligolactides. Polylactic acids are usually synthesized in three ways: condensation/binding of lactic acid, azeotropic distillation of water, and polymerization of lactide with the opening of its cycle. The macrocycles introduced into the reaction medium played the role of improving agent. Besides, they provide products with additional binding sites, control fixed orientation for the spatial arrangement of the polymer chains and alter hydrophilic-lipophilic balance. All these properties make it possible to implement the principles of molecular and supramolecular recognition, when functional groups and binding sites of the modified polylactide specifically bind functional groups of biopolymers and response on such interaction by changing the structure and aggregation properties detected by optical and other signal transducers.
When deposited on the surface of a sensor, modified polylactides form films whose permeability, charge and sorption properties are determined by the “macrocyclic knot” of the polymer. By varying the thiacalixarene conformation, it is possible to selectively “tune” the properties of the coating so that it binds biopolymers but retains high permeability for small ions. Such properties made it possible to obtain a kind of universal sensors that respond to analyte molecules bonded to a bioreceptor on the sensor interface. The signal might be a change in the flow of ions added to the sample. It is decreased in the analyte binding or increased in the introduction of compounds charged oppositely to a probe ion. This approach was implemented in a family of electrochemical sensors, which, depending on the nature of the bioreceptor, made it possible to detect the presence of a wide variety of compounds - from toxins (aflatoxins, organophosphorus pesticides) to biological additives - antioxidants and vitamins. In this case, it did not matter whether analyte molecules were able to oxidize at the electrode as required by “ordinary” electrochemical sensors, or not.
By varying the nature of the modified polylactide, first of all, the conformation of the “macrocyclic knot”, it is possible to select the conditions for the inclusion of the receptor, either synthetic or natural, and record its interactions with molecules with high sensitivity. Different composite materials based on oligolactides as matrices for such redox probes, approaches to their synthesis, and examples of sensors based on them were described in this mini-review.
“Polylactic acid modified by “macrocyclic knots” gives a wide variety of materials characterized by high chemical stability, biocompatibility, ease of synthesis and the possibility of directional binding to a wide range of compounds demanded in clinical analysis, biomedicine in general, and the manufacture of portable biosensors for point-of -care diagnostics,” says Professor Stoikov. “Prospects for programming properties that are promising for assembling of drug delivery systems, photosensitizers in photodynamic therapy, and protein binding are also obvious. It is also important that the mentioned achievements require implementation of very simple algorithms, the elucidation of which is an important part of the project, no less than the synthesis of individual polymers. This includes the choice of the type of macrocyclic block, stoichiometry of the polycondensation reaction, method of introducing the polymer into the sensitive layer of a sensor and the process of membrane formation with the inclusion of biocomponents. It sounds trivial, but the confirmation of these laws required the use of all modern tools of physical organic chemistry and electrochemical methods of analysis. Some of the described advantages have already been shown by the examples of electrochemical sensors and biosensors with advanced characteristics of the determination of drugs, metabolites, antioxidants, aflatoxins, Alzheimer's disease medications, assessment of total antioxidant activity, etc.”
The derivatives of macrocyclic compounds are already used in pharmacology as antibacterial and catalytic systems, components of drug delivery and release agents, and as materials for biosensors. The introduction of such polyfunctional fragments into the structure of synthetic polymers allows modeling active sites of various enzymes depriving them of their main drawback, i.e., instability, but maintaining high selectivity of a substrate binding. DNA sensors are a promising tool required in personalized medicine to provide diagnostic information outside the hospital. Thus, potential directions for further work include development of new chemical (electrochemical) sensors and biosensors based on poly- and oligolactic acids modified with macrocyclic fragments (porphyrin, cyclodextrin, and cyclophane).
Modification of Oligo- and Polylactides With Macrocyclic Fragments: Synthesis and Properties
https://www.frontiersin.org/articles/10.3389/fchem.2019.00554/full
Olga A. Mostovaya, Vladimir V. Gorbachuk, Pavel L. Padnya, Alena A. Vavilova, Gennady A. Evtugyn and Ivan I. Stoikov