Combustion behavior of aromatics may provide keys to enhancing heavy oil extraction
Kazan University’s In-Situ Combustion Lab continues its inquiries in Journal of Industrial and Engineering Chemistry.
The problem of petroleum depletion becomes more and pertinent every day. As deficits arise, non-traditional and heavy oils, including bitumen and shales, emerge as the focus of extensive research. Globally, they account for about 60 to 70 percent of explored reserves. For Russia, it’s also over 60 percent.
Head of Ecooil Research Unit at Kazan University Mikhail Varfolomeev comments on this particular paper, “We studied how components and composition of oil can influence the implementation of in-situ combustion. We used model components to mimic in-situ processes and compiled our recommendations for oil companies.”
For this purpose, the team separately researches saturated fractions, aromatic fractions, tars, and asphaltenes.
Senior Research Associate of Rheology and Thermochemical Research Lab Yuan Chengdong explains, “We managed to compare the characteristics of these four components and analyze the effects of their combined combustion. The study helps to better understand the behavior of crude oil during in-situ combustion. We can understand the mechanisms of hydrocarbon oxidation because alkanes, aromatics and their oxygen-containing and sulfur-containing derivatives can be found in common motor fuels, such as petrol, diesel, and airplane fuel.”
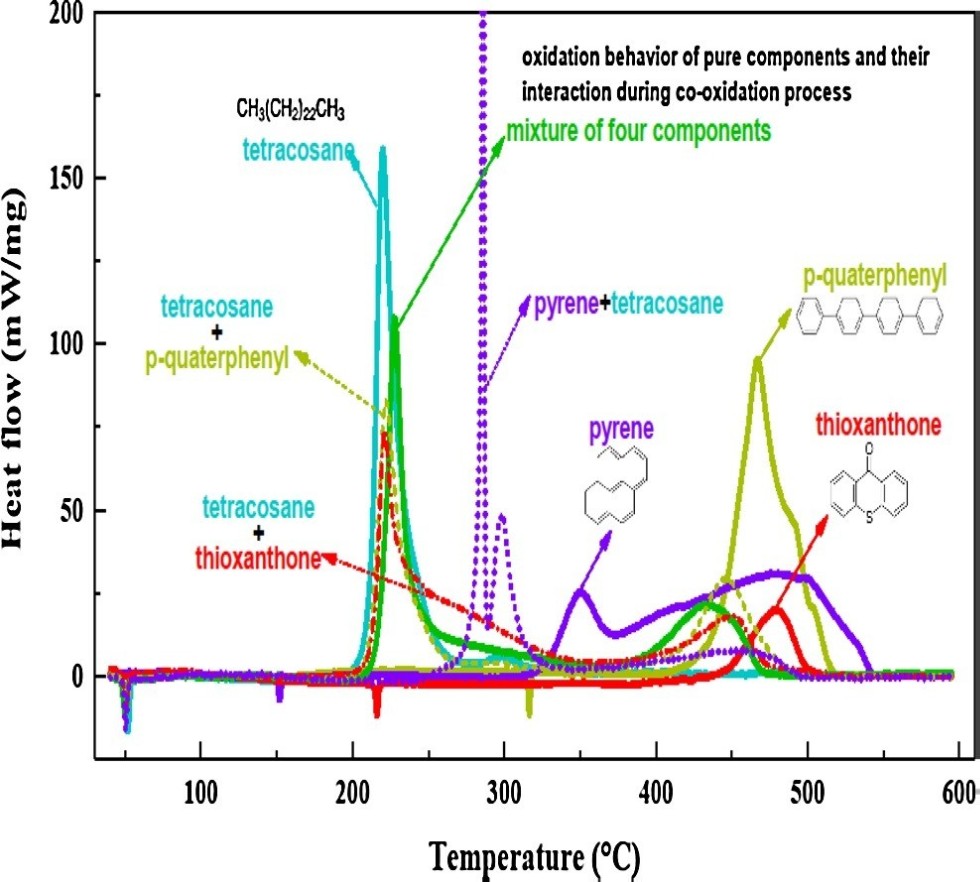
The combustion behavior of aromatics (p-quaterphenyl, thioxanthone, pyrene) and their interaction with n-alkane (tetracosane) were investigated by high-pressure differential scanning calorimetry (HP-DSC). Tetracosane only showed low-temperature oxidation (LTO), while p-quaterphenyl and thioxanthone only showed high-temperature oxidation (HTO). Pyrene exhibited a unique middle-high temperature oxidation (M-HTO). Tetracosane significantly promoted the HTO of p-quaterphenyl and thioxanthone, and shifted their HTO into lower temperatures. While p-quaterphenyl and thioxanthone did not significantly affect the occurrence of the LTO of tetracosane, but they did reduce the heat release and reaction rate of the LTO of tetracosane. The co-oxidation of tetracosane and pyrene triggered an intense interaction that exerts a strong inhibition on the LTO of tetracosane, and induces an explosive oxidation reaction followed by a mild oxidation from 280 to 325 °C. The intense interaction also significantly promoted the HTO of the pyrene. In general, the interaction strength is in turn pyrene + tetracosane > thioxanthone + tetracosane > p-quaterphenyl + tetracosane. Due to the strong interaction between the alkane and aromatics during their co-oxidation, the additivity of heat release in both LTO and HTO cannot be applied in terms of reaction process as well as total heat release.
Combustion behavior of aromatics and their interaction with n-alkane in in-situ combustion enhanced oil recovery process: Thermochemistry
https://www.sciencedirect.com/science/article/pii/S1226086X19301753
Chengdong Yuan, Dmitrii A.Emelianov, Mikhail A.Varfolomeev, Mustafa Abaas
Source text: Adelya Shemelova
Translation: Yury Nurmeev