Research projects
In the center of biomedical microscopy following projects are performed:
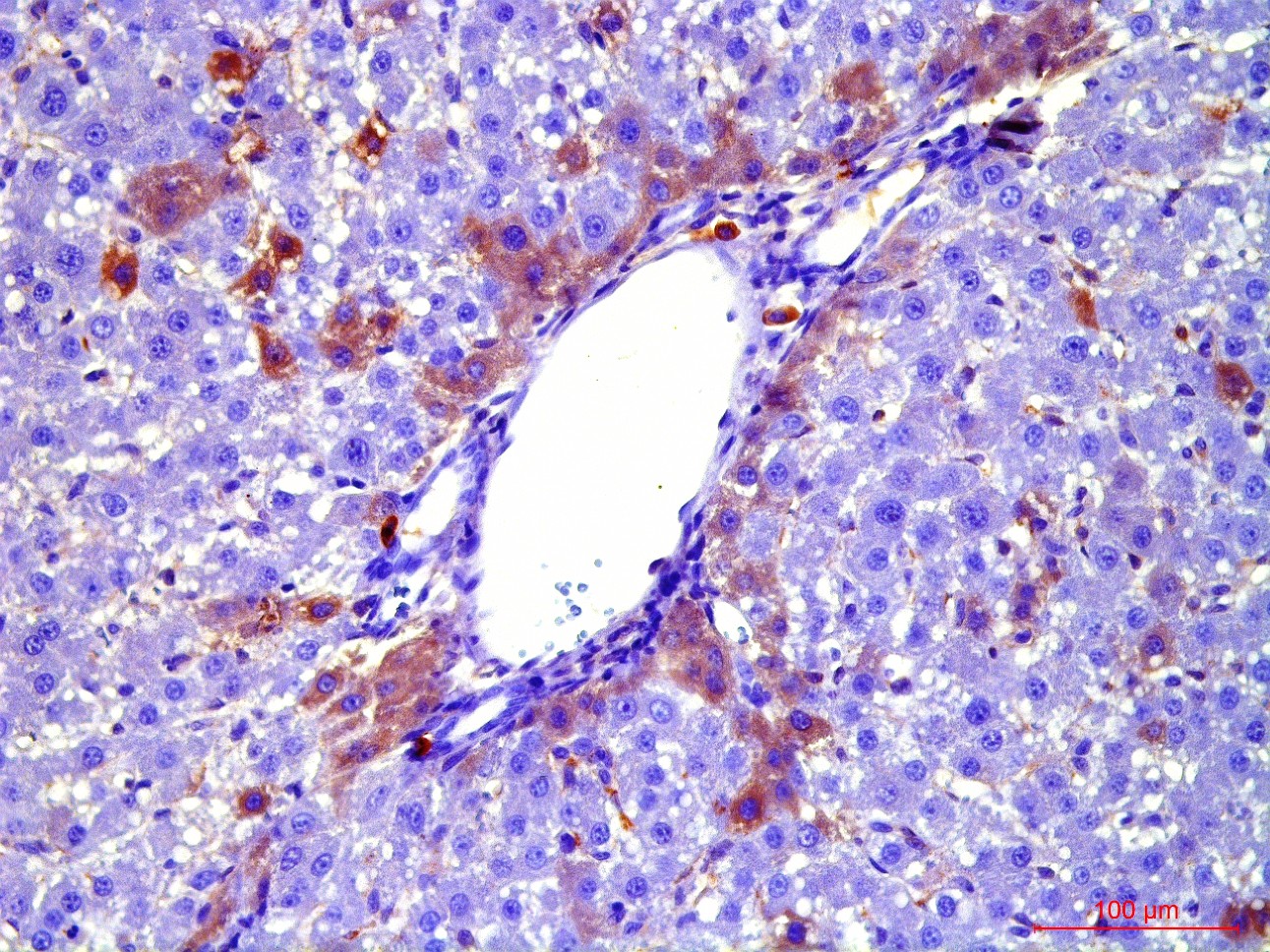
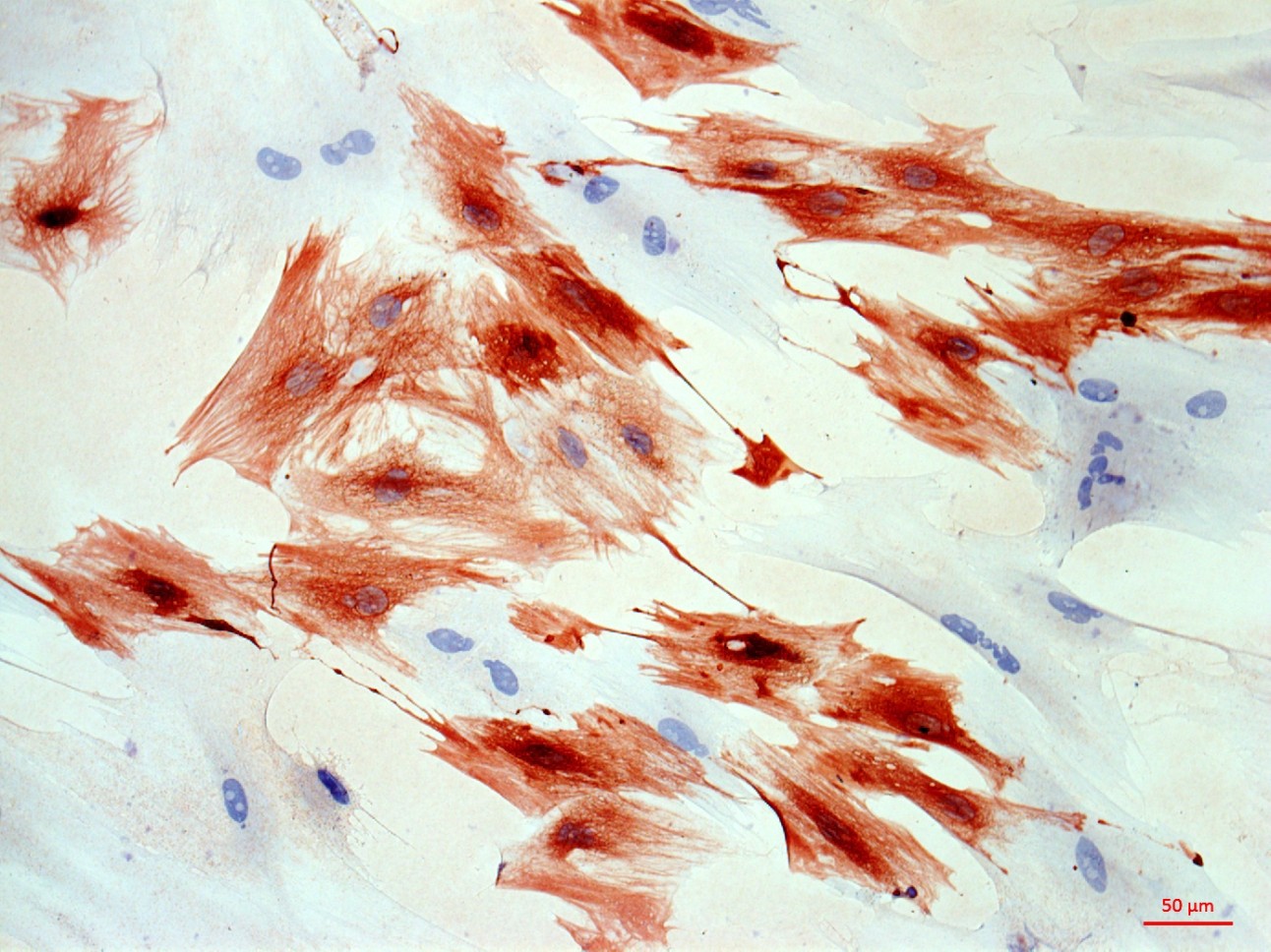
At the first stage of the research the maximum number of cells with the phenotype of hepatocytes, bearing the mark of transplanted liver stellate cells (HSC) GFP, was shown after the transplantation of freshly isolated HSC to the rats with partial hepatectomy (PH). Transplantation of HSC after PH has advantages over the transplantation of umbilical cord blood monocytes in the number of donor hepatocytes differentiated from the transplanted cells as well as in the time of their appearance. However, in case of toxic liver damage by carbon tetrachloride (CTC), the number of hepatocytes differentiated from transplanted HSC regardless of their preparation was considerably less than that in the PH model. The largest number of hepatocytes expressing green fluorescent protein GFP was recorded after transplantation of HSC activated in vivo. It was also shown that transplanted HSC do not differentiate into cholangiocytes like human cord blood mononuclear cells do. HSC transplantation significantly reduces the activation of regional stem cell compartment during regeneration on the studied models of liver damage.
Transplantation of GFP-positive HSC after PH and introduction of acetylaminofluorene (AAF) gives rise to GFP-positive hepatocytes and sinusoidal cells in the recipient's liver. In the case of CTC+AAF liver damage the appearance of GFP-positive sinusoidal cells, hepatocytes, and single cholangiocytes were shown. It was found that transplantation of both native and activated HSC is effective for the hepatocytes repopulation. It also stimulates liver regeneration in the experimental models of liver damage with AAF blocking of hepatocyte proliferation which is evidenced by the appearance of GFP +, α-fetoprotein + cytokeratin 19+ hepatocytes. The most significant stimulatory effect of HSC transplantation was observed after PH. Transplantation of HSC activated in vivo or in vitro, did not reveal significant differences between the groups. It was established that native HSC are more effective for recovery of the liver after PH+AAF, whereas in vivo activated HSC are more effective for regeneration after CTC+AAF. It is important that transplantation of freshly isolated as well as activated in vivo or in vitro HSC do not cause differentiation of endogenous and transplanted HSC into myofibroblasts, thus it is safe in terms of liver fibrosis.
Recombinant adenovirus, co-expressing hepatocyte growth factor (HGF) and fibroblast growth factor (FGF4), which was then successfully used for the transduction of HSC, was received as a result of the project. Transplantation of HSC transduced with genes of red fluorescent protein RFP, HGF and FGF4 to intact rats confirmed their viability and showed that these genes facilitate engraftment of cells and stimulate their ability to differentiate into hepatocytes. Repopulating effect of HSC transduced with therapeutic genes HGF and FGF4 was significantly higher and longer compared with transplantation of cells transduced with GFP only into animals after PH with and without the AAF. However, in case of CTC liver damage with and without the introduction of AAP, transplantation of RFP + / HGF + / FGF4 + HSC had no stimulatory effect on hepatocyte repopulation and liver regeneration. HSC transduced by therapeutic genes HGF and FGF4 stimulate transient HSC transdifferentiation into myofibroblasts with no signs of fibrosis.
In general, the results of this study confirmed the hypothesis of regional stem cell compartment in the sinusoids of the liver, the ability of transplanted HSC differentiate into hepatocytes and their dual influence on the process of regeneration (stimulation of recipient cells and differentiation of transplanted cells). The study showed that genetic and cellular therapy is a promising source of liver regeneration and has no risks associated with the development of liver fibrosis.
The results of the research were published in the English language monograph, six articles in the Scopus journals and more than 16 abstracts presented at International congresses and Russian conferences.
 Peripheral arteries disease of lower limbs is characterized by decreasing of arteries lumen which leads to ischemia of muscle tissue and development of chronic arterial insufficiency. This disease often leads to a disability of active and able-bodied population. Traditional methods of treatment are ineffective because there is a damage of the smallest vessels. In recent years therapeutic angiogenesis is gaining more and more interest. It is based on stimulation of development of new vessels and capillaries by means of recombinant inductors of angiogenesis, injection of cellular therapy and genetic vectors encoding growth factors. However, the early clinical trials of therapeutic angiogenesis showed limited efficiency of gene and cellular therapy.
Peripheral arteries disease of lower limbs is characterized by decreasing of arteries lumen which leads to ischemia of muscle tissue and development of chronic arterial insufficiency. This disease often leads to a disability of active and able-bodied population. Traditional methods of treatment are ineffective because there is a damage of the smallest vessels. In recent years therapeutic angiogenesis is gaining more and more interest. It is based on stimulation of development of new vessels and capillaries by means of recombinant inductors of angiogenesis, injection of cellular therapy and genetic vectors encoding growth factors. However, the early clinical trials of therapeutic angiogenesis showed limited efficiency of gene and cellular therapy.
The reason for it is that there are still not studied fundamental mechanisms of post-ischemic regeneration of muscular tissue and angiogenesis. Detailed information on a chronological sequence of participation of various cell types and growth factors in the course of formation of new vessels and regeneration of muscular fibers in the conditions of ischemia is practically absent. Interactions between different factors causing regeneration of muscle tissue and formation of new vessels (paracrine effects of transplanted cells, differentiation into cells of muscular tissue and vessels, fusion with cells) during gene and cellular therapy of chronic ischemia remain unknown.
During the project we are planning to perform surgical models of chronic ischemia of lower extremities on rats with visualization of a volumetric blood supply, carrying out functional tests for an assessment of lower limbs function, morphological analysis of lower limb muscles, visualization of ischemia degree, analysis of growth factors expression with Western blotting and RT-PCR. On the basis of received information, we are planning to select a combination of therapeutic genes, design two-cassette plasmids expressing the therapeutic gene and green fluorescent protein. Then we intend consecutively according to the stage of growth factors action transplant plasmids or bone marrow hematopoietic stem cells transfected with corresponding plasmids, into muscles of lower limbs of rats with chronic ischemia and assess blood supply restoration, functional tests, histological changes in regenerating tissue.
 The analysis of results will allow to answer a question about the role and sequence of different growth factors and cell types participation in post-ischemic muscle regeneration and to offer an optimum sequence and the timing for application of therapeutic genes for therapy of chronic arterial insufficiency and to find out mechanisms of chronic arterial insufficiency.
The analysis of results will allow to answer a question about the role and sequence of different growth factors and cell types participation in post-ischemic muscle regeneration and to offer an optimum sequence and the timing for application of therapeutic genes for therapy of chronic arterial insufficiency and to find out mechanisms of chronic arterial insufficiency.