Cell surface engineering
Directed and controllable modification of live cell membranes and cell walls is deemed to be an important tool to enhance or attenuate the intrinsic properties of the cells. We have developed a set of versatile techniques to interface human and microbial cell with polyelectrolyte coatings, magnetic and metal nanoparticles, carbon and halloysite nanotubes, and hard mineral shells. These coatings enable cells to perform functions often completely different from their original specialisation. These cells are routinely termed as “cyborg cells”, i.e. natural live core coated with artificial nanostructurised shell. We have applied “cyborg cells” to fabricate whole cell biosensors, toxicity microscreening devices, tissue engineering and fabrication of artificial multicellular clusters. Our current research is focused on several important directions: 1) fabrication of artificial tissue-like clusters using magnetically-modified human cells; 2) fabrication of “smart” cell surface-modifying coatings based on responsive polymers and functional nanoparticles and nanotubes.

Nanotoxicology
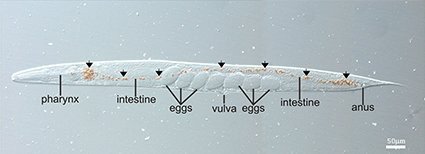
Nanotoxicology is an emerging area of research aimed at finding out the potential toxic effects of nanomaterials on humans and ecosystems. The application of in vivo models in testing the toxicity of nanoparticles is currently regarded one of the promising way to investigate the effects of nanomaterials on living organisms. We employ a number of invertebrate models to assess the toxicity of nanomaterials. Recently, we introduced a novel method to deliver nanomaterials into Caenorhabditis elegans nematodes, based on using nanoparticle-coated microbial cells as “nanobait”. These nanobaits are ingested by nematodes as a sole food source via pharyngeal pumping, which results in localization of nanoparticles inside the digestive tract of the worms. We applied our delivery method to test the toxicity of silver and magnetic nanoparticles, and halloysite nanotubes. Delivery of nanoparticles results in magnetic labelling of living nematodes, rendering them magnetically-responsive. Currently, we are working on elucidation of toxicity mechanisms of clay nanoparticles and evaluate other less conventional invertebrate models.

Tissue engineering
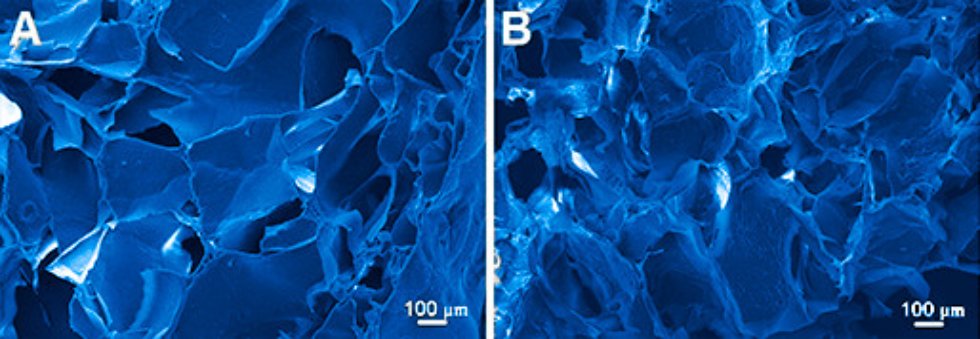
Tissue engineering is a dynamically developed and popular sector of biomedicine. Demand for replacement of lost or damaged tissues and organs is enormous and can not be fully satisfied by the transplantation of donor organs. In this regard, the prototyping of natural tissues is an actual problem. An important component of artificially created tissue is extracellular matrix as the structure which is essential for the proper functioning of cells. The approach for creating matrixes proposed in our laboratory characterized by the absence of crosslinkers which can be toxic to cells, and also we used the introduction of nanoparticles into the matrix. The obtained nanocomposite scaffolds exhibit shape memory upon deformation and have a porous structure suitable for cell adhesion and proliferation. The study of mechanical properties and in vitro / in vivo analysis of the 3D nanocomposite porous hydrogels from natural biopolymers doped with nanoparticles revealed their biocompatibility and biodegradability.

Targeted drug delivery
Targeted drug delivery - the actual direction of pharmacology, which aims to create drug delivery systems that would allow the active agent to reach a target organ or cells. In our laboratory it has been proposed to use natural clay mineral halloysite nanotubes as nanocontainers. Its unique feature - the existence of the internal cavity, which can be filled with different compounds - making halloysite nanotubes promising material for nanocontainers. Using different biological objects low toxicity of halloysite was shown. In addition we studied the uptake of halloysite nanotubes by different types of cell lines. We have found that different cells have different "appetite" in relation to the nanotubes. This phenomenon can be used to create drug delivery systems for various types of tissues. We have also developed a method of functional stoppers formation at the ends of the nanotubes to prevent exposure to the drug agent before the reaching the target tissue.